|
|||||||||||
State Space Modeling of the electrochemistry at the solid-gas interfaces
Contact: Michel Prestat
The mechanisms and kinetics of solid oxide fuel cell electrode reactions (oxygen reduction and fuel oxidation) have been investigated for several years in the SOFC group. A widespread tool to investigate those reactions is electrochemical impedance spectroscopy. However the interpretation of experimental impedance spectra fitted with equivalent circuits in terms of reaction steps and rate constants turns out to be very difficult. An alternative approach consists in calculating the faradaic impedance of an electrochemical reaction of interest and comparing it with the experimental impedance.
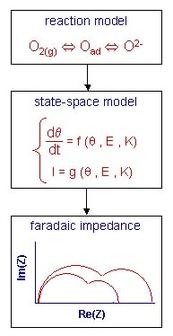
The calculation of faradaic impedances can be done numerically with State-Space Modeling, whose principle is schematically described on Figure 1. The mass transport and charge transfer equations are derived from the proposed reaction model. The resulting system of equations (called "state-space model") is linearized and undergoes a Laplace transform. This yields the faradaic impedance that can be studied as a function of all the rate constants of the model and of the experimental parameters such as cathode potential and oxygen partial pressure. The use of a Matlab/Simulink package software enables to calculate impedances even for sophisticated models that cannot be treated analytically.
The faradaic impedance is a function of a vector K, whose components are the rate constants of the proposed reaction model. A model is validated when simulated and experimental impedances exhibit a good agreement for different experimental conditions and for one unique vector K (pratical identification).
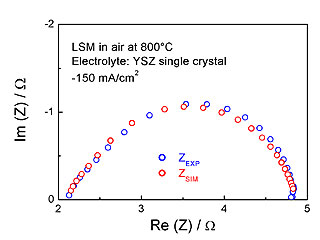
This approach has been applied to oxygen reduction at SOFC electronic conducting cathodes such as LSM (strontium substituted lanthanum manganite). In this case, oxygen reduction is supposed to take place exclusively at the surface of the cathode (no bulk reaction pathway). In a recent study, we have showed, with a combination of simulations and experimental measurements of electrochemical impedances, that oxygen reduction involves a mixed control of oxygen adsorption and charge transfer (see figure 2).
Current research is now focusing on reactions with more complex mechanisms and kinetics such as oxygen reduction at mixed ionic-electronic conductors, where a competition occurs between surface and bulk reaction pathways.
For further information please consult the works of Mitterdorfer, Bieberle, Prestat in our literature database.
Wichtiger Hinweis:
Diese Website wird in älteren Versionen von Netscape ohne
graphische Elemente dargestellt. Die Funktionalität der
Website ist aber trotzdem gewährleistet. Wenn Sie diese
Website regelmässig benutzen, empfehlen wir Ihnen, auf
Ihrem Computer einen aktuellen Browser zu installieren. Weitere
Informationen finden Sie auf
folgender
Seite.
Important Note:
The content in this site is accessible to any browser or
Internet device, however, some graphics will display correctly
only in the newer versions of Netscape. To get the most out of
our site we suggest you upgrade to a newer browser.
More
information