|
|||||||||||
Experimental determination of heterogeneous phase equilibria in multi-component systems
Contact: Ming Chen
A fundamental goal in materials science is to be able to control the final properties of a material, may it be physical, mechanical or chemical properties. To achieve this, one must understand the interrelations between the chemical composition, the processing, the microstructure, and the properties of the final material. During processing, and often also during use, most materials undergo one or more heterogeneous reactions, or rather phase transformations. Knowledge of heterogeneous equilibria, is obviously, essential for the development and processing of materials.
Our group has been studying heterogeneous phase equilibria since 1990's, using X-ray diffraction techniques, thermal analyses (DTA and TG), and microstructure analyses (SEM, TEM, and EDX). The experimental work done so far has been focused on the following issues:
Phase equilibria in high-temperature superconducting materials
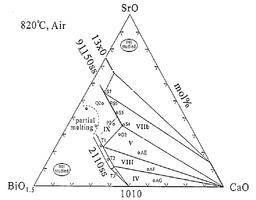
The Bi-Sr-Ca-Cu-O system contains several superconducting phases, including Bi-2212 and Bi-2223. These phases are of great technological interest since they have high critical temperatures (Tc: 95 K for Bi-2212 and 115 K for Bi-2223) and can be relatively easily synthesized. The most common method to prepare high quality components of Bi-2212 is the partial melt-process. It was found that a homogeneous microstructure with highly aligned grains leads to high critical current densities and the secondary phases may have a deleterious influence on the Bi-2212 formation and the grain alignment.
Bi-2212 is usually processed on silver substrates since silver is chemically stable at high temperature and has no detrimental effect on the superconducting properties. During the processing of Bi-2212 thick films on silver substrates, it has been observed that Ag lowers the melting temperature of the 2212 phase by about 30 K.
Till now, we have experimentally investigated the following systems:
- Bi-Sr-Ca-O
- Sr-Ca-Cu-O
- Ag-Bi-O
- Ag-Sr-Cu-O and Ag-Ca-Cu-O
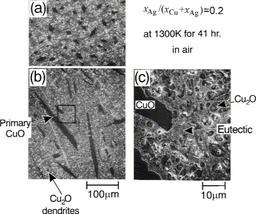
- Ag-Cu2O-CuO
Phase equilibria in materials for solid oxide fuel cells (SOFC)
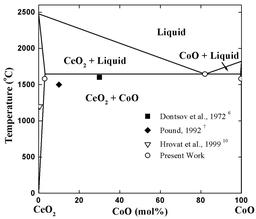
Ceria-based ceramics are very attractive as solid electrolytes for developing low temperature SOFCs and low temperature oxygen sensors. One of the drawbacks of ceria-based ceramics is the high sintering temperature. By doping with a small amount of Co3O4 (less than 5 cat.%), we attained dense Ce0.8Gd0.2O2-x (CGO, >99% theoretical density) at temperatures below 900 °C, with an average grain size of less than 150 nm. The knowledge of phase equilibria in the CeO2-CoO system would be helpful to understand the sintering mechanism of CoO doped CGO.
We investigated the CeO2-CoO system. A eutectic reaction was found at a temperature of 1645 °C and the evaluated eutectic point is at 82 mol% CoO.
For more information please consult in our literature database the papers of Assal, Buhl, Cantoni, Chen, Grundy, Hallstedt, Kleinlogel, Müller, and Suzuki.
Wichtiger Hinweis:
Diese Website wird in älteren Versionen von Netscape ohne
graphische Elemente dargestellt. Die Funktionalität der
Website ist aber trotzdem gewährleistet. Wenn Sie diese
Website regelmässig benutzen, empfehlen wir Ihnen, auf
Ihrem Computer einen aktuellen Browser zu installieren. Weitere
Informationen finden Sie auf
folgender
Seite.
Important Note:
The content in this site is accessible to any browser or
Internet device, however, some graphics will display correctly
only in the newer versions of Netscape. To get the most out of
our site we suggest you upgrade to a newer browser.
More
information