|
|||||||||||
Single Chamber Solid Oxide Fuel Cells
contact: Brandon Bürgler
Single Chamber Solid Oxide Fuel Cells (SC-SOFC) can be understood as Fuel Cells with only one gas compartment (thus, "single chamber"). In conventional Fuel Cells of any type, the oxidant and the fuel are usually separated by a gas tight electrolyte. The cell has two gas chambers. The operation of a SC-SOFC relies on the selectivity of the cathode and the anode which are both exposed to the same gas mixture of fuel and air.
Differences to conventional Solid Oxide Fuel Cells
In conventional SOFCs there is only one reaction that is possible at the cathode, i.e. the reduction of oxygen. At the anode the oxidation of the fuel occurs. Thus, the electrode materials do not need to be selective for the anode or cathode reaction. They only need to have a high activity for the reaction taking place. However, in SC-SOFC apart of the high activity, the anode needs to be selective for the oxidation of the fuel whereas the cathode needs to exhibit selective reduction of oxygen and inertness to the fuel. The generation of an open circuit voltage (OCV) is due to the difference of catalytic activity. It is obvious that a cell with two electrodes made of the same materials can not generate electric power. Highly active standard materials used in conventional SOFCs like La0.8Sr0.2MnO3-x (LSM) La0.8Sr0.2Co0.2Fe0.8O3-x (LSCF), Ni-Zr0.8Y0.2O1.9 (Ni-YSZ) are not applicable in SC-SOFC because they do not exhibit high enough selectivity.
In principle the reactions at the two electrodes are the same for both types of SOFCs. Even so, in the SC-SOFC the selectivity of materials allows the omission of the gas separating membrane.
Principle of operation
The electrolyte is commonly referred to an ionic conductor, however, vacant oxygen lattice sites are the veritable charge carriers. Let us, thus, assume oxygen vacancies (VO**) to be the charge carrying, mobile species. The anode is the site where the oxidation of the fuel takes place. The fuel (hydrogen) and oxygen surface atoms (OOx) are consumed. Water, oxygen vacancies and electrons (e-) are created at the anode. The following reaction Equation describes what is happening at the anode:
H2 + OOx = H2O + VO** + 2 e-
At the cathode the reduction of the oxygen from air occurs, consuming the electrons that have flown through an electrical circuit and have emerged at the anode by the oxidation of the fuel. Oxygen vacancies are annihilated at the cathode by the reaction with gaseous oxygen. For every annihilated vacancy a pair of electrons is consumed. This reaction leads to the formation of regular oxygen ions in the crystal lattice at the surface of the electrolyte.
1/2 O2 (g) + 2 e- + VO** = OOx
It can be easily verified that the sum of the two reactions corresponds to the combustion reaction of hydrogen with water as the product. The electrons of the spatially separated redox reactions are forced to flow through an external circuit that includes the electric load (any electric power-consuming device). The electrodes are sites, where the charge carrying specie is converted from electrons to vacancies or vice versa.
At too high temperature (>700°C) both electrode materials act as a single phase combustion catalyst and the OCV vanishes. The introduction of ceria as low temperature electrolyte was thus a mandatory step for SC-SOFC to give reasonable power output.
Layout and Materials
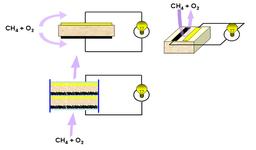
Figure 1 shows the three basic and novel layouts possible with SC-SOFCs. The cells always have three basic components, electrolyte, anode and cathode. At the electrodes the electric current must be collected. This is done with so called current-collectors. The three cell designs are:
- sandwich structure (opposite electrodes on intermediary electrolyte )
- side by side electrodes (not possible with conventional fuel cells)
- fully porous cells (porous interconnects are represented in light blue)
The materials used for SC-SOFC are Sm0.5Sr0.5CoO3-x (SSC) with perovskite structure as the cathode and Ce0.9Gd0.1O1.95 (CGO) with fluorite structure as the electrolyte. A composite material of metallic Nickel and CGO is used as highly active anode (Ni-CGO). The microstructure of an anode can be seen in Figure 2.
Advantages
SC-SOFC offer the following advantages:
- No separation of fuel and oxidant is needed. Complex flow field structures can be eliminated.
- sealing of cell from surroundings is easily achieved.
- A variety of geometries apart from planar and tubular known from conventional fuel cells is possible.
- Electrolyte must not be gas tight. Fully porous cells can be fabricated.
- Connection of several cells in parallel and in series on the same side of the electrolyte. Generation of practically useful voltages and currents without the need for a complex cell stack.
- The "Single Chamber" approach also offers advantages for miniaturisation. Half the gas flow channels are needed. No micro sealing is necessary. Furthermore, it is very difficult to make separated gas chambers on the micrometer scale.
Challenges
- The operation of the cell relies on the selectivity of the electrodes. Conventional fuel cell materials like LSM, LSCF and Ni-YSZ are unsuitable because they only have a high catalytic activity without the necessary selectivity.
- Mixture of fuel and oxygen can be explosive.
- Fuel utilisation is lower than in conventional fuel cells.
- Parasitic, nonelectrochemical reactions will decrease efficiency.
Research
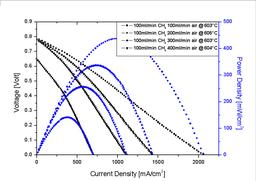
We investigate alternative materials, fabrication processes, operating conditions and performance of SC-SOFCs with simple geometry. The performance of a cell at 600° C can be seen in Figure 3. The maximum power density at 100 ml/min methane and 200 ml/min air was approximately 450 mW/cm2. We also investigate the influence of Co-doped electrolytes on the fuel cell performance. We seek to understand how selective electrodes work, how we can improve the performance of SC-SOFC and what materials are best suited. The dependence of OCV and maximum power density on temperature, gas flows, gas compositions and geometry is also subject to current work.
Literature
- Hibino, T. et al., A Low Operating Temperature Solid Oxide Fuel Cell in Hydrocarbon-Air Mixtures. Science, 2000. 288:2031-2033.
- Hibino, T. et al., Improvement of a Single Chamber Solid Oxide Fuel Cell and Evaluation of New Cell Designs. Journal of the Electrochemical Society, 2000. 147(4):1338-1343.
Wichtiger Hinweis:
Diese Website wird in älteren Versionen von Netscape ohne
graphische Elemente dargestellt. Die Funktionalität der
Website ist aber trotzdem gewährleistet. Wenn Sie diese
Website regelmässig benutzen, empfehlen wir Ihnen, auf
Ihrem Computer einen aktuellen Browser zu installieren. Weitere
Informationen finden Sie auf
folgender
Seite.
Important Note:
The content in this site is accessible to any browser or
Internet device, however, some graphics will display correctly
only in the newer versions of Netscape. To get the most out of
our site we suggest you upgrade to a newer browser.
More
information