|
|||||||||||
Thermodynamic Modeling using the Calphad Method
Contact: Nicholas Grundy, Ming Chen
The basic idea
A fundamental goal in materials science is to be able to control the final physical, mechanical or chemical properties of a material. In order to do that one must understand the interrelations between the raw materials and their chemical composition, the processing conditions, the microstructure (in a wide sense) thus produced and the properties of the final material. During processing, and often also during use, most materials undergo one or more heterogeneous reactions, or rather phase transformations. This is particularly obvious in melt processing where the material is completely or partially melted and then solidified.
To aid understanding of the interrelation between chemical composition, processing conditions and microstructure phase diagrams are used extensively in the development and processing of metals and ceramics and also in geochemistry. Experimentally determined phase diagrams are usually only available for binary systems, to some extent for ternary systems and very rarely for higher order systems. Since a phase diagram is a representation of the thermodynamic properties of a system it is in principle possible to calculate the phase diagram if the thermodynamic properties are known. It is then also possible to extrapolate to multicomponent systems.
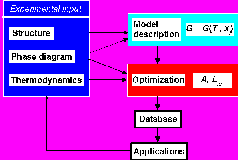
The CALPHAD method
By combining knowledge on the phase diagram and the thermodynamic properties of a system a model description of the system suitable for phase diagram calculations can be created. Descriptions of low order systems can be combined to make extrapolations to higher order systems. For this purpose the Gibbs energy of each phase is described by a suitable model containing a relatively small number of variable coefficients. These coefficients are optimised using experimental information on phase equilibria such as melting temperatures, other transformation temperatures, solubilities and on thermodynamic properties such as heat capacities, enthalpies of formation, chemical potentials from emf or pressure measurements. Most of this information is taken from the literature, but we can also do own phase equilibria studies using typically light optical microscopy, SEM/EDX, EPMA, XRD and DTA/TG. There is a large number of thermodynamic models available and we normally use the very general "compound energy formalism" for solid solution phases and the "two-sublattice model for partially ionic liquids" for liquid phases.
Bi-based superconductors
The Bi-Sr-Ca-Cu-O system contains the two important superconducting phases Bi-2212 and Bi-2223. We have modelled the Ag-Bi-Sr-Ca-Cu-O system in order to study melt processing of Bi-2212 with and without Ag additions. Bi-2223 is practically impossible to process without the addition of Pb and we plan to add Pb to the above description as well.
La-Sr-Mn-O perovskites for SOFC cathodes
Perovskites from the La-Sr-Mn-O system (LSM) are used as cathode material in yttria stabilised zirconia (YSZ) electrolyte solid oxide fuel cells (SOFC). Under certain conditions LSM reacts with YSZ to form insulating phases which degrade the performance of the fuel cell. LSM itself is an electronic conductor, but by adding e.g. Co, Ni or Fe the oxygen ion conductivity can be increased, which could lead to improved performance of the SOFC. At the same time the tendency to form insulating phases at the YSZ interface increases. In the present work the La-Sr-Mn-O system is to be modelled and combined with the Y-Zr-O system in order to study the conditions for formation of insulating phases in detail. Additions of Co, Ni and/or Fe are also planned.
For further information on this research area please consult in our literature database the papers of Assal, Chen, Grundy, Hallsted, Risold.
Wichtiger Hinweis:
Diese Website wird in älteren Versionen von Netscape ohne
graphische Elemente dargestellt. Die Funktionalität der
Website ist aber trotzdem gewährleistet. Wenn Sie diese
Website regelmässig benutzen, empfehlen wir Ihnen, auf
Ihrem Computer einen aktuellen Browser zu installieren. Weitere
Informationen finden Sie auf
folgender
Seite.
Important Note:
The content in this site is accessible to any browser or
Internet device, however, some graphics will display correctly
only in the newer versions of Netscape. To get the most out of
our site we suggest you upgrade to a newer browser.
More
information